Boron Tribromide A Reagent In Organic Synthesis Borates Today

Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of boron tribromide, BBr 3. The electron-pair geometry is trigonal-pyramidal, the molecular geometry is trigonal-pyramidal. The electron-pair geometry is trigonal-planar, the molecular geometry is trigonal-planar.
Boron Buy Boron Supplements

(ii) Draw a 'dot-and-cross' diagram to show the bonding in a boron tribromidemolecule. Show outer electronsonly. (d) State whether the following substances conduct electricity when solid or molten, and explain your answers in terms of the particlesinvolved: • aluminium • aluminium fluoride • boron tribromide.
Thermo Scientific Chemicals Boron tribromide, 99+, Thermo Scientific Chemicals Fisher Scientific

Introduction. Boron tribromide is a versatile reagent utilized in diverse areas ranging from polymer chemistry to natural product synthesis. 1 Owing its high reactivity to the Lewis acidic boron center, BBr 3 reactions include haloborylation, 2 boron-silicon exchange, 3 and rearrangement of 7,7-diphenylhydromorphone derivatives. 4 While there is no shortage in the diversity of BBr 3-mediated.
Purchase Boron tribromide 1M in Dichloromethane [10294334] online • Catalog • Molekula Group

ED7400000. Molecular Formula BBr. Average mass 250.523 Da. Monoisotopic mass 247.764297 Da. ChemSpider ID 23479.
BORON TRIBROMIDE LR, 500gm Bottle at best price in Mumbai ID 2851886086873

Chemical Formula: BBr3. Melting Point: -46.3. Boiling Point: 91.3 C. Density: 2.60 g/mL. Boron tribromide (BBr3) is a Lewis acid commonly used for the demethylation of methyl ethers. Boron tribromide is available neat, but is often purchased as a 1M solution in either DCM, hexane, or heptane. The most common of these is the 1M solution in DCM.
Boron Tribromide Molecular Structure Isolated on White Stock Illustration Illustration of

The researchers selected boron tribromide (BBr 3) as a bromide radical donor (Br ), since the B-O bond that forms upon radical generation using O 2 is much stronger than the B-Br bond that breaks, making the process thermodynamically favourable. They applied this approach to investigate the hydrobromination of cyclopropanes, for the novel and selective formation of the anti-Markovnikov.
Boron tribromide, ≥99.9 10294334 Manufacturers & Suppliers in India with worldwide shipping.

Boron tribromide. Formula: BBr 3. Molecular weight: 250.523. IUPAC Standard InChI:InChI=1S/BBr3/c2-1 (3)4 Copy. IUPAC Standard InChIKey:ILAHWRKJUDSMFH-UHFFFAOYSA-N Copy. CAS Registry Number: 10294-33-4. Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or.
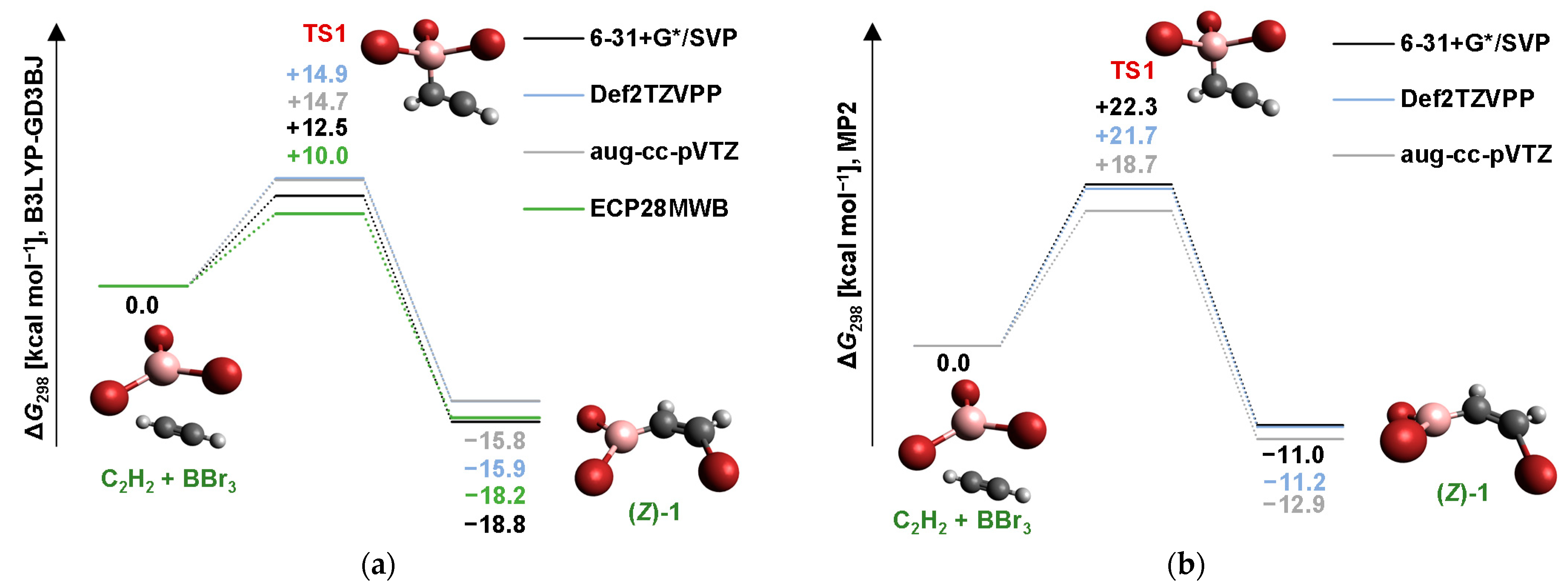
Molecules Free FullText Free Radical Isomerizations in Acetylene Bromoboration Reaction
Boron tribromide (BBr 3) is a strong Lewis acid generally used as a reagent for the deprotection of ethers.Alkyl aryl ethers are cleaved at the alkyl-oxygen bond to give ArOH and alkyl bromides. In a particular case, BBr 3 was used to cleave acetals that could not be deprotected under standard acidic conditions. Similarly, amino acid-protecting groups such as benzyloxycarbonyl and tert.
Boron tribromide 25g Alchemist Elements

The Lewis dot structure of Boron trichlortde enhances the idea about sharing electrons. Boron has three valance electrons and three chlorine atoms have (3*7= 21) valance electrons. Therefore, total amount of valance electrons take place in the formation of BCl3 is 24. The central atom boron shares each of its three electrons with the individual.
【サイズ】 bbrg3の通販 by ミツジ's shop|ラクマ カテゴリ www.bran.it

Monograph ID M2619 Title Boron Tribromide Molecular formula BBr 3 Molecular weight 250.52 Percent composition B 4.32%, Br 95.69%
Scientific Chemical Boron Tribromide Manufacturer from Hyderabad

A detailed mechanism illustrating demethylation of methyl ethers using boron tribromide (BBr3). Boron Tribromide (BBr3) Mechanism - Demethylation of Methyl Ethers only search this site
Is BBr3 (Boron tribromide ) Ionic or Covalent/Molecular? YouTube

Borob Tribromide reacts vigorously with water to generate gaseous HBr. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Bromide gas will be created in 0.18 minutes. Experimental details are in the following: "Development of the Table of.
boron tribromide cas 10294334 Haihang Industry

Colour: colourless. Appearance: liquid. Melting point: -46°C. Boiling point: 91°C. Density: 2600 kg m -3. The following are some synonyms of boron tribromide: boron tribromide. boron (III) bromide. The oxidation number of boron in boron tribromide is 3.
CAS No.10294334,Boron tribromide Suppliers,MSDS download
The typical preparation of the cyclic boroles from acyclic starting materials usually involves acid base chemistry or transacetalization protocols. 1,3,2-Diazaborolidines, such as 311 and 313, are readily available via the reaction of the corresponding amines 310 and 314 with boron tribromide (BBr3) and boron halide 312 ( Scheme 51) <2006JCD3777>.
Boron Tribromide BBr3 Image & Photo (Free Trial) Bigstock
Boron tribromide is a chemical compound of boron and bromine. It is a commercially available strong Lewis acid. Boron tribromide is an excellent demethylating or dealkylating agent for ethers and is often used in the production of pharmaceuticals. Additionally, it also finds applications in olefin polymerization and in Friedel-Crafts chemistry.
Boron tribromide Boron triiodide Fosfor tribromide Kimya donan şeffaf PNG görüntüsü

Boron tribromide, BBr 3, is a colorless, fuming liquid compound containing boron and bromine. Commercial samples usually are amber to red/brown, due to weak bromine contamination. It is decomposed by water and alcohols. [2]
.

